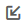Inhibition of Angiopoietin-Like Protein 3 With Evinacumab in Subjects With High and Severe Hypertriglyceridemia
Hypertriglyceridemia is associated with increased risk of atherosclerotic cardiovascular disease and acute pancreatitis.
Sequence variations in the lipoprotein lipase (LPL) gene and other LPL pathway genes result in severely elevated triglycerides. Angiopoietin-like protein 3 (ANGPTL3) inhibits LPL activity, increasing plasma triglycerides and low-density lipoprotein cholesterol (LDL-C). Evinacumab, an ANGPTL3 inhibitor, has been shown to reduce triglycerides and cholesterol in healthy volunteers and subjects with homozygous familial hypercholesterolemia.
In a phase 1, single-dose study of evinacumab ( NCT01749878), individuals with mixed dyslipidemia with triglycerides ≥150-450 mg/dL (Cohort A) receiving single ascending doses of evinacumab experienced sustained reductions in triglycerides and cholesterol. Here we report results from 2 other cohorts of the same study.
The study protocol was approved by appropriately constituted Institutional Review Boards (IRBs) (University of Pennsylvania IRB, University of Texas Southwestern Medical Center IRB, and the Research and Development Committee at the Corporal Michael J. Crescenz VA Medical Center) and independent ethics committees (Integ Review Ethical Review Board and MidLands Independent Review Board).
Cohort B subjects (n = 7) had moderate hypertriglyceridemia (≥450 and <1,500 mg/dL) and received intravenous (IV) evinacumab 10 mg/kg (n = 5) or placebo (n = 2). Cohort C subjects (n = 9) had LPL pathway sequence variations (sequence variants identified in apolipoprotein A5 [APOA5], apolipoprotein C2 [APOC2], and LPL) and severe hypertriglyceridemia (>1,000 mg/dL), and received evinacumab 250 mg subcutaneous (SC) (n = 4), evinacumab 20 mg/kg IV (n = 2), placebo SC (n = 2), or placebo IV (n = 1). Safety up to day 126 and changes in lipids were analyzed.
Treatment-emergent adverse events (TEAEs) occurred in 100.0% and 81.8% of placebo- and evinacumab-treated subjects, respectively. No deaths or TEAEs leading to discontinuation were reported. In the severe hypertriglyceridemia cohort, 3 of 6 evinacumab-treated subjects reported ≥1 serious TEAE (hospitalization for alcoholic pancreatitis complicated by alcohol withdrawal and acute respiratory distress syndrome; hospitalization for acute pancreatitis; and transient ischemic attack followed by 3 hospitalizations for recurrent pancreatitis); none were considered treatment-related (occurring ≥70 days after study drug administration).
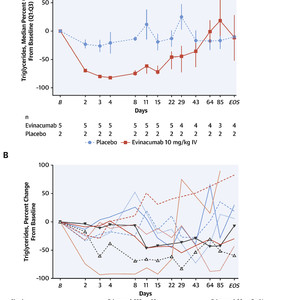
Evinacumab-treated subjects with moderate hypertriglyceridemia experienced marked triglyceride reductions, with a peak median reduction of 81.8% at day 4 vs 20.6% with placebo (Figure 1A) (median attained levels were 83.0 mg/dL [min 78, max 113 mg/dL] vs 444.0 mg/dL [min 349, max 539 mg/dL], respectively). Mean very-low density lipoprotein cholesterol (VLDL-C) levels were reduced with evinacumab until approximately day 64, with a peak reduction of 82.2% from baseline at day 4 versus 0.8% with placebo. At day 4, absolute mean ± SD VLDL-C levels were 19.6 ± 2.9 mg/dL and 47.5 ± 31.8 mg/dL with evinacumab and placebo, respectively. Triglyceride and VLDL-C treatment differences in change from baseline demonstrated nominal P values of 0.0952 over days 2-11 (P values for descriptive purposes only). A mean increase in LDL-C was observed with evinacumab, persisting until days 29-43, with a peak of approximately 54.4% from baseline at day 4 versus 5.5% with placebo.

Triglyceride Values Over Time in Subjects With Moderate and Severe Hypertriglyceridemia
Patients with severe hypertriglyceridemia exhibited wide-ranging responses to evinacumab with triglyceride reductions of 0.9%-93.2% on day 3, sustained until day 22 in most subjects (Figure 1B). Evinacumab IV 20 mg/kg induced a maximum mean reduction in VLDL-C of 64.9% (108.0 mg/dL; n = 1) versus 42.0% (177.0 mg/dL; n = 1) with placebo on day 22, sustained until the end of the study, whereas evinacumab SC 250 mg resulted in a maximum reduction of 37.8% (mean absolute value 261 mg/dL [SE 85.4]; n = 4) versus an 18.4% increase (361.0 mg/dL; n = 1) with placebo on day 8, sustained until day 22. Evinacumab groups showed a mean percent increase in LDL-C with a maximum increase of 74.4% with evinacumab IV 20 mg/kg versus a 12.5% reduction with placebo on day 29, and a maximum increase of 79.6% with evinacumab SC 250 mg versus a 53.4% reduction with placebo on day 64.
The observed increases in LDL-C with evinacumab in both cohorts were not unexpected, and were likely caused by enhanced conversion of VLDL and intermediate-density lipoprotein particles to low-density lipoprotein particles.
The wide-ranging responses observed with evinacumab in the severe hypertriglyceridemia cohort may be attributable to variability of disease-causing sequence variations in the LPL pathway (results not shown).
Current treatment for hypertriglyceridemia focuses on diet, supplementation with omega-3 fatty acids, and fibrates, particularly if there is increased risk of acute pancreatitis. However, these approaches have limited efficacy. Our results indicate that evinacumab was generally well-tolerated, and substantially reduces triglycerides and VLDL-C in hypertriglyceridemic subjects.
This article is reproduced from JACC journals.
surgerycast
Shanghai Headquarter
Address: Room 201, 2121 Hongmei South Road, Minhang District, Shanghai
Tel: 400-888-5088
Email:surgerycast@qtct.com.cn
Beijing Office
Address: room 709, No.8, Qihang international phase III, No.16, Chenguang East Road, Fangshan District, Beijing
contact number:010-5123-5010 13331082638(Liu Jie)