Transcatheter Treatment of Concomitant Aortic Stenosis and Tricuspid Regurgitation: Are We There Yet?
Identification of more than moderate secondary (functional) tricuspid regurgitation (TR) in a patient with severe, symptomatic aortic stenosis (AS) who is a candidate for transcatheter aortic valve replacement (TAVR) is not unusual. In most of the teams, it currently does not question TAVR indication, based on the assumption that it may regress once the left-sided heart valve disease is cured, and in case of residual significant TR, that it should not have the same life-threatening potential in the short or mid term as AS.
In this issue of JACC: Cardiovascular Interventions, Tomii et al present a retrospective analysis of Bern University Hospital TAVR registry in which 2,641 consecutive patients were prospectively enrolled from 2007 to 2019. They focused on TR severity assessment before and after TAVR, potential eligibility for transcatheter tricuspid valve intervention (TTVI) after TAVR, and their relationship with 1-year outcome. They confirm a global trend, already reported regarding TR evolution after TAVR, with more than 50% of patients experiencing a TR reduction, whereas in 5% to 7% of cases, TR worsens after aortic valve replacement. An important finding is that post-TAVR severe and massive TR (and not baseline TR severity) is associated with a 2-fold increased risk of all-cause death and a 2- to 3-fold increased risk of cardiovascular death at 1 year. Among patients still having more than moderate TR after TAVR, the authors isolated a small group deemed potential candidates for TTVI. Another original finding is to show that those patients had a 2-fold increased risk of all-cause and cardiovascular death between 30 days to 1 year after TAVR with a poor functional status.
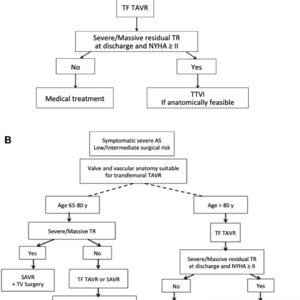
This study has some limitations, clearly acknowledged by the authors. Among those, potential indication for TTVI was in fact assessed by a combination of functional status and residual severity of TR after TAVR, corresponding to TRILUMINATE (Trial to Evaluate Treatment With Abbott Transcatheter Clip Repair System in Patients With Moderate or Greater Tricuspid Regurgitation) inclusion criteria. This does not entail anatomical feasibility of TTVI, which is purely anatomically determined mainly by transesophageal echocardiography and cardiac computed tomography scan measurements, and whose criteria vary according to the device (eg, edge-to-edge tricuspid valve repair, tricuspid annuloplasty, transcatheter tricuspid valve replacement).
But this observation immediately raises the hypothesis of potential functional and prognostic improvement of this group of patients by TTVI. Of course, this is currently purely speculative, and there is yet a long journey before we can confirm such clinical benefit. However, the data we have so far are promising. In the recently published TRILUMINATE trial, 1-year outcomes after tricuspid valve repair with the TriClip system (Abbott Vascular) were reported, and it was found to be safe (overall major adverse event rate and all-cause mortality both at 7.1% in a high-risk population) and effective with significant functional improvement and TR reduction to moderate or less in 71% of patients. Transcatheter tricuspid valve replacement (TTVR) is emerging with extremely encouraging results. In a multicenter, observational first-in-human experience, TTVR with the EVOQUE valve (Edwards Lifesciences) in a small group of 25 patients was technically feasible in 92% of cases, with 0% procedural or 30-day mortality, New York Heart Association functional class I or II for 76% of patients (as opposed to 96% New York Heart Association functional class III or IV at baseline), and TR grade ≤2+ in 96% at 30 days. The next step will be to confirm the superiority of TTVI other optimal medical therapy regarding effectiveness, without major safety issues, by randomized controlled trials. This is work in progress with both TRI-FR (Multicentric Randomized Evaluation of Tricuspid Valve Percutaneous Repair System [Clip for the Tricuspid Valve] in the Treatment of Severe Secondary Tricuspid Disorders) (for TriClip) ( NCT04646811) and TRISCEND II (Edwards EVOQUE Transcatheter Tricuspid Valve Replacement: Pivotal Clinical Investigation of Safety and Clinical Efficacy Using a Novel Device) (for EVOQUE TTVR [ NCT04482062]) trials recruiting.
Based on this, we could imagine how guidelines on severe, symptomatic AS treatment could evolve integrating concomitant TR (Figure 1). This is again purely speculative, and time will tell whether it is realistic forecasting.

Speculative Adaptation of Aortic Stenosis Treatment Guidelines Integrating Concomitant Tricuspid Regurgitation
In April 2022, we will celebrate the 20th anniversary of the first TAVR performed by Alain Cribier and his team in Rouen University Hospital. Let’s take this opportunity to express to him our gratitude for having revolutionized treatment of severe AS. Twenty years later, after a rigorous validation by randomized controlled trials in each stratum of surgical risk, transfemoral TAVR has been granted a class 1A recommendation for symptomatic patients with severe AS without anatomic contraindication, from 65 years of age. Among controversies that arose from those American College of Cardiology/American Heart Association guidelines release, one was related to the concern that this recommendation could be interpreted as a blind authorization for interventional cardiologists to favor TAVR instead of surgical aortic valve replacement, without any discrimination. Oppositely, I consider that it reinforces the need for the Heart Team to master all the subtleties of preoperative selection, necessarily involving proficiency sharing from all its members, aiming for patient’s benefit as unique objective. Even though only hypothesis-generating, the results presented by Tomii et al are adding another level of granularity in the decision-making process to select the most appropriate therapy for patients with severe AS, and again are putting the Heart Team at the core of patient’s care.
This article is reproduced from JACC journals.
surgerycast
Shanghai Headquarter
Address: Room 201, 2121 Hongmei South Road, Minhang District, Shanghai
Tel: 400-888-5088
Email:surgerycast@qtct.com.cn
Beijing Office
Address: room 709, No.8, Qihang international phase III, No.16, Chenguang East Road, Fangshan District, Beijing
contact number:010-5123-5010 13331082638(Liu Jie)